Votre question concerne quel type d'offre ?
Votre question concerne quel couloir Ségur ?
Votre question concerne quel dispositif Ségur ?
Votre question concerne quel produit ou service produit?
Votre question concerne quelle thématique ?
The study conducted by the ANS helped establish that the equipment used in telehealth practices can be :
- A space dedicated to telehealth
- A teleconsultation booth
- A console with screen
- A teleconsultation cart
- A visioconference cart
- A PACS console
- A computer with webcam
- A digital tablet
- A smartphone
- Carte Vitale reader
- Digital dermatoscope
- Digital otoscope
- Digital ophthalmoscope
- Digital fundus camera
- Connected echograph scanner
- Connected thermometer, stetoscope, scale and tensionmeter
Further information is available in the Documentation.
Cette réponse vous a-t-elle été utile ?
There are two steps to follow:
Step 1: Administrative phase
Should you wish to order test CPS cards or register authorisations for test software, you may:
- read our complete products offer ;
- place an order
To place an order, select your Profile and your Structure, then choose “Produits de développement. Commander des produits de développement : carte et/ou certificat logiciel de test" (Test products for products, cards and/or test software).
Step 2: Technical phase
Use the card you validated after step 1 in order to connect with the Trusted Platform IGC-Santé. You will be able to order, withdraw, monitor and revoke test certificates through the IHM or Webservice interface.
In order to do so, make sur you have inserted your test card in the card reader.
Read more about specific setup guidelines:
Cette réponse vous a-t-elle été utile ?
In an inclusive way, all digital services aimed at citizens and useful for their healthcare can apply. Eligible services must be for the use of patients/citizens and proposed by public or private actors in the medical (health, wellness), social-medical, or social fields. The services must be included the definition set out by the L. 1111-13-1 bill:
These services may include mobile apps, websites, connected objects, medical devices. They may be free services or not.
Services in the realm of the EU regulation 2017/745 relating to medical devices must be conform to security and performance requirements. If your solution passes the conformity requirements, it will receive the CE label for medical devices.
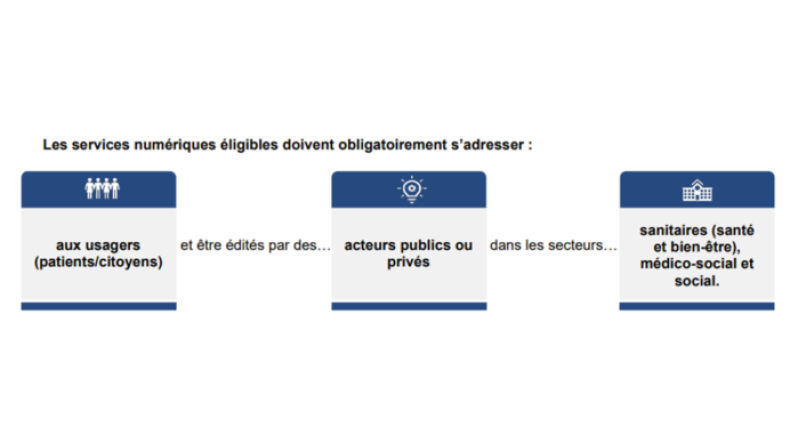
Cette réponse vous a-t-elle été utile ?
To register on the Convergence platform, you need to download the account request form to open an account as a company representative.
Before completing this form, please check that your company is not already represented by another person who is able to create an account for you to access the Platform. If you have any questions, please contact support at ans-support-convergence@esante.gouv.fr.
As soon as you receive the e-mail confirming the creation of your account, you will be able to initialize your password and access the Platform after accepting the General Terms of Use.
Cette réponse vous a-t-elle été utile ?
The IGC-Santé is dedicated to the health sector and follows strict procedures in terms of data collection, professional identification, and works with certified authorities (RPPS register, etc.).
The certificates issued by the IGC guarantee the security of software or electronic cards, such as the CPS card.
The IGC also manages the publication of these certificates and can revoke them – this is signalled to the apps using certificates in revocation listings.
Cette réponse vous a-t-elle été utile ?
There are two main reasons for the creation of IGC-Santé :
- guaranteeing the security of private keys and certificates issued by the ANS: the access to these private keys must be limited in order to prevent duplicates or their installation more than one device;
- maintaining a continuity in services: many health apps used to work on certificates issued by former CKI that ceased their activity in January 2021. These apps must be compatible with certificates issued by the IGC-Santé.
In addition, these certificates meet the security standards (risk analysis, safety policies), or “Certifying Policies” that comply with the PGSS-IS guidelines.
Cette réponse vous a-t-elle été utile ?
You may divide your service into as many products as you wish to register on the platform. The appropriate segmentation must correspond to your activity and the roadmaps/processes available on the platform.
It is important to include all the different versions associated with support or devices as different products, as they could impact the maturity level assessment of your solution.
In order to make the identification of products easier, each edition and version of the commercialised products needs to be registered. We suggest you use this format to name your product - (Name of the Product) (OS) (Device) - with the following terms:
- device can be named “Mobile” or “Website”
- OS can be named “iOS”, “Android”, “Windows”, “Linux” or any other operating system.
In addition, for registration into Mon Espace Santé (My Health Space): you must add as a product the solution that will be visible to the citizen/user in the service catalogue. The commercial name must be the same as the name visible by the citizen.
Cette réponse vous a-t-elle été utile ?
The Convergence platform supports you in developing your business and defining your plan for compliance with regulations specific to the e-health sector.
To this end, the platform is organized around two tracks:
- digital health doctrine ;
- My health space.
Each of these paths is broken down into steps:
- doctrine is divided into 4 steps: Evaluation and projection socles , Teleconsultation, teleexpertise, telecare;
- My Health Space is divided into 2 steps: referencing without data exchange and referencing with data exchange.
The procedures include questionnaires which, once completed, will enable you to assess the maturity and/or compliance of your products with the digital health doctrine, or the assessments required for My Health Space registration.
Cette réponse vous a-t-elle été utile ?
The first step in completing the platform is to identify a manager who will oversee the completion operations. This person can then make an inventory of the products to be included in the scope of data entry, and list the resources to be mobilized: product managers, technical and functional experts, etc. Despite efforts to contextualize the questions, knowledge of healthcare IS interoperability, security and the ambitions of the doctrine of digital health is a prerequisite.
The questionnaires can then be read in groups. Based on the completion of an initial product, the reading of the questionnaires enables the identification of subjects that have been mastered, as well as those that require further investigation.
The pilot can then propose regular meetings with all product managers to monitor the progress of data entry, guarantee the consistency of responses and share expert feedback.
Cette réponse vous a-t-elle été utile ?
There are two main user profiles on the Convergence platform:
- Manager: this is either the Corporate Officer or a person authorized by the Corporate Officer; he/she is authorized to add new contributors, create products and grant rights to contributors from his/her company;
- Contributor: authorized to create accounts for other contributors, create products and complete questionnaires, within the limits of the rights assigned to him/her.
Contributors are given access rights to questionnaires for products already created within the platform, and to questionnaires relating to global strategy.
Contributors who do not have the right to validate questionnaires can only save drafts for questionnaires to which they have access.
Cette réponse vous a-t-elle été utile ?
In order for your service to be referenced in the My Health Space database, it must comply with the eHealth doctrine.
The doctrine is re-edited each year and defines the base rules of good practice for security, interoperability, and ethics applicable to eHealth services.
Cette réponse vous a-t-elle été utile ?
My Health Space includes the following health data:
- The medical profile entered by the patient, in an unstructured format (preconditions, allergies);
- Health measurements (weight, height, BMI, temperature, waist size, heart rate, blood pressure, glycemic index, level of pain) in FHIR format;
- Health documents (for example: test and diagnosis results, hospitalisation reports, medical treatments).
Cette réponse vous a-t-elle été utile ?


