Votre question concerne quel type d'offre ?
Votre question concerne quel couloir Ségur ?
Votre question concerne quel dispositif Ségur ?
Votre question concerne quel produit ou service produit?
Votre question concerne quelle thématique ?
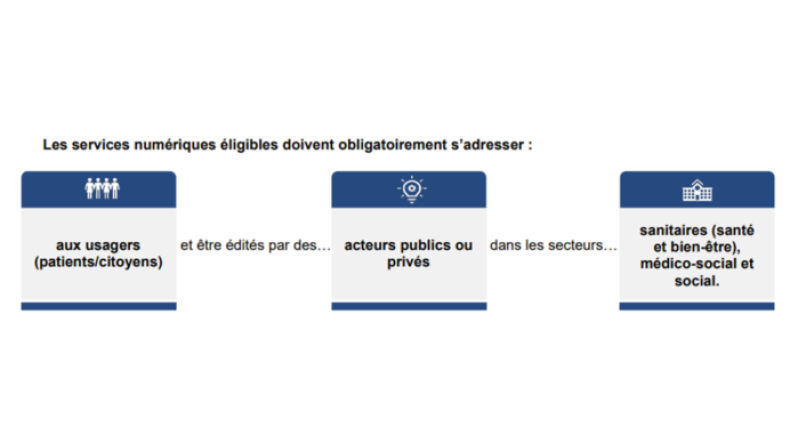
In an inclusive way, all digital services aimed at citizens and useful for their healthcare can apply. Eligible services must be for the use of patients/citizens and proposed by public or private actors in the medical (health, wellness), social-medical, or social fields. The services must be included the definition set out by the L. 1111-13-1 bill:
These services may include mobile apps, websites, connected objects, medical devices. They may be free services or not.
Services in the realm of the EU regulation 2017/745 relating to medical devices must be conform to security and performance requirements. If your solution passes the conformity requirements, it will receive the CE label for medical devices.
Cette réponse vous a-t-elle été utile ?
Your application needs to be complete and signed, with all the following documents:
- the MDPH label rules, which describes the labelling process
- the convention regarding the use of the label, which defines the procedures that take place after the label is granted. For example: obligations tied to the label, audit visits, cases of modifying the label, etc.
- the annexes to the convention;
- the license of use for the labelled solution;
- the insurance security plan for the conformity checks relating to the MDPH label;
- if applicable, the decisions issued by partner organisations dealing with exchange fluxes (CNAF/SNGI, Imprimerie Nationale, etc.) to attest you are using the correct one.
The application must be sent in paper AND via electronic mail to:
Agence du Numérique en Santé
Labellisation logiciels Maisons Départementales des Personnes Handicapées
9 rue Georges Pitard 75015 Paris
labellisation.mdph@esante.gouv.fr
Cette réponse vous a-t-elle été utile ?
These documents are made available by the CNDA as a .zip file below.
Cette réponse vous a-t-elle été utile ?
Generally speaking, the PGSSI-S needs to be applied as soon as personal health data are being handled. It is relevant to the public sector as well as the private sector, health professionals, workers of the social-health and social sectors, healthcare establishments and service providers.
As a patient, the PGSSI-S is a seal of guarantee on the accountability of digital health ecosystems.
Cette réponse vous a-t-elle été utile ?
Complying with the PGSSI-S frames of reference is either required by law (if the documents have been approved by a ministerial decree) or meant to be followed on a short-term basis until the documents are approved by the ministry.
Cette réponse vous a-t-elle été utile ?
The application for the SI-MDPH label is open to any legal entity with a software solution aimed at the departmental disability centres (MDPHs). This includes the MDPHs themselves, which develop their own solutions in conjunction with the relevant Departmental Council.
The label application is a voluntary process: Anyone can be a candidate if they feel that their solution complies with the current requirements and the scope of the sector’s functional standards.
Cette réponse vous a-t-elle été utile ?
In order for your service to be referenced in the My Health Space database, it must comply with the eHealth doctrine.
The doctrine is re-edited each year and defines the base rules of good practice for security, interoperability, and ethics applicable to eHealth services.
Cette réponse vous a-t-elle été utile ?
My Health Space includes the following health data:
- The medical profile entered by the patient, in an unstructured format (preconditions, allergies);
- Health measurements (weight, height, BMI, temperature, waist size, heart rate, blood pressure, glycemic index, level of pain) in FHIR format;
- Health documents (for example: test and diagnosis results, hospitalisation reports, medical treatments).
Cette réponse vous a-t-elle été utile ?


