Votre question concerne quel type d'offre ?
Votre question concerne quel couloir Ségur ?
Votre question concerne quel dispositif Ségur ?
Votre question concerne quel produit ou service produit?
Votre question concerne quelle thématique ?
All the resources for funding are available in our Ségur section for digital equipment.
Cette réponse vous a-t-elle été utile ?
Health professionals are encouraged to use MOS and NOS for three main reasons :
- enhance the interoperability of information systems by harmonising names, attributes, codes and nomenclatures;
- share the same understanding of the information, regardless of which directory or reference document it is taken from;
- facilitate the specification, analysis and conception of a project.
The ANS offers training on MOS-NOS and the elaboration method for exchanges’ functional specs (see our Documentation section).
The illustration below gives you examples of the types of professionals using the MOS-NOS:
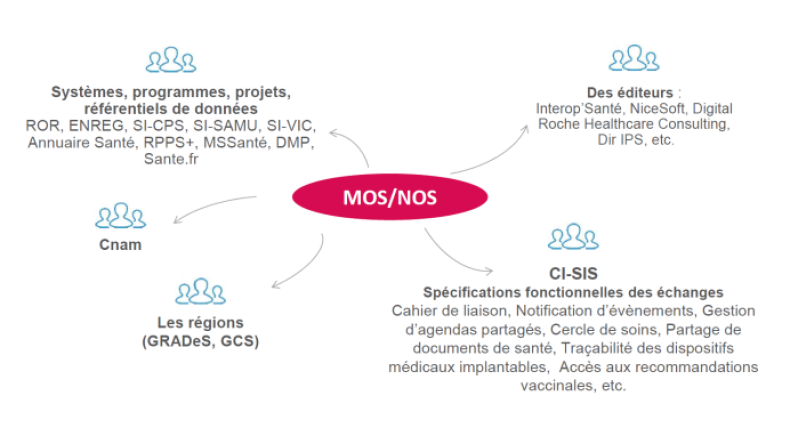
Cette réponse vous a-t-elle été utile ?
For your application to be complete, you need to include several credentials and documents.
The list of documents and credentials needed is detailed in the Application form (download it below). You will need to send and application for each product you wish to label.
You must return your application form with all the supporting material by email AND via postal services:
- the complete application form needs to be sent by mail to:
Agence du numérique en santé (ANS)
Labellisation logiciel Maisons et Centres de Santé
9, rue Georges Pitard
75015 Paris - follow up with sending digital versions (PDF scans) of the agreement and its annexes, in two copies, signed and completed to:
Cette réponse vous a-t-elle été utile ?
The MOS is a collection of concepts described homogeneously and neutrally in terms of technologies. It offers a common description of the information processed and exchanged in the information systems to make digital communication easier.
The overall consistency of the MOS is founded on the definition and description of its UML (Unified Modelling Language) concepts.
Some MOS concepts can be coded. They are associated with the nomenclatures of health objects (NOS), i.e. lists of codes/labels.
You are encouraged to use MOS and NOS to :
- optimise and coordinate efforts when you analyse or conceive a system (or an application) by re-using the same semantic components;
- make sure there is consistency in your internal developments and with external systems, for the best possible interoperability.
Cette réponse vous a-t-elle été utile ?
The European Commission’s studies have determined there is a need for a base vocabulary, such as MOS and NOS, which can be used as a starting point for:
- develop and evolve information systems (IS) to formalise the conceptual and logical data they utilise (for instance, the MOS is a reference for the modelling of the RPPS);
- share information between ISs to create specific models of data they can use (thus, the MOS is underlying the ROR exposure model);
- combine and synthetise elements originating from different sources;
- publish data in a common format, such as a directory or catalogue of service (for example, the Annuaire Santé / Health Pro Directory).
Cette réponse vous a-t-elle été utile ?
When your software gets the label attribution, the successful candidate will receive by email the visual elements you will need to communicate about it.
The editor of the solution (or the solution range) will receive the label after signing the agreement with the ANS. They will be able to use the label on commercial publications, technical documents, with no particular restriction on the type of format used (paper, Internet, intranet, etc.)
The recipient must mention the level of the label (1 or 2) they obtained. These specifications are set out in the Functional referential (in our Documentation section)
Cette réponse vous a-t-elle été utile ?
There are several levels of requirements for a module’s integration into the Hospital Information System.
The main requirements set out by the referential are:
- Interfacing, control, security guidelines;
- Prescription process requirements;
- National prescription thesaurus integration requirements;
- Requirements regarding medico-economic and decision processes;
- Guidelines for ergonomics, functions, and notification alerts;
- Settings function requirements.
In total, 139 requirements are used to reach the minimal level of security needed for a solution’s integration into the hospital information system.
Cette réponse vous a-t-elle été utile ?
The current referential (2017) was written with industry experts. It introduces the particular context of this mission and the goals to reach in order to improve safety measures in neonatology and paediatric reanimation. The referential walks you through the fundamental concepts you need to grasp to understand the several requirements and protocols involved with software development for this sector.
Cette réponse vous a-t-elle été utile ?
Neonatology is a high-risk practice for two main reasons:
- The patients are extremely fragile (premature babies)
- 50% of the drugs used in the sector have yet to receive a marketing authorisation.
A survey conducted in 2014 evidenced a risk in the prescription process across the sector. The digitalisation of prescription is among the 41 recommendations that the report issued in order to increase safety levels.
Cette réponse vous a-t-elle été utile ?


