Votre question concerne quel type d'offre ?
Votre question concerne quel couloir Ségur ?
Votre question concerne quel dispositif Ségur ?
Votre question concerne quel produit ou service produit?
Votre question concerne quelle thématique ?
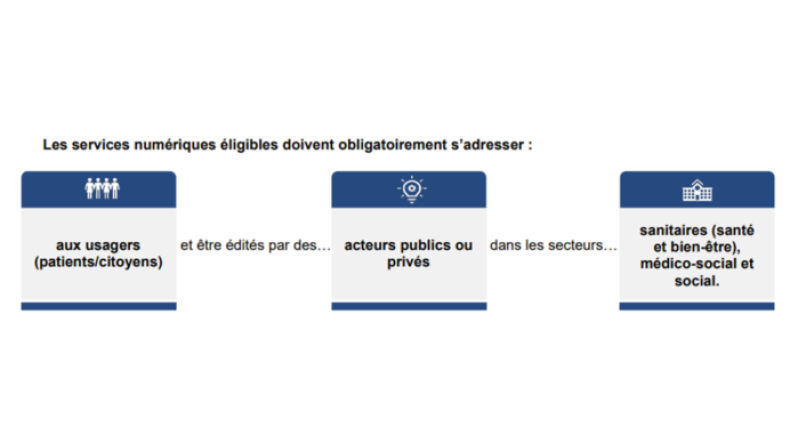
In an inclusive way, all digital services aimed at citizens and useful for their healthcare can apply. Eligible services must be for the use of patients/citizens and proposed by public or private actors in the medical (health, wellness), social-medical, or social fields. The services must be included the definition set out by the L. 1111-13-1 bill:
These services may include mobile apps, websites, connected objects, medical devices. They may be free services or not.
Services in the realm of the EU regulation 2017/745 relating to medical devices must be conform to security and performance requirements. If your solution passes the conformity requirements, it will receive the CE label for medical devices.
Cette réponse vous a-t-elle été utile ?
Your level of access to the ROR data depends on the nature of the data you wish to use (public/restricted) and the perimeter you wish to cover (one ROR or several ROR directories).
The ROR data in public access:
- is available as opendata since mid-2022;
- will be available directly on the national ROR with no specific procedure in early 2023.
The ROR data in restricted access (professional access):
- for one regional ROR, you must complete a request form to join the regional circle of trust and send it to the region’s GRADeS;
- for several ROR directories, you must complete a request form to join the national circle of trust and send it to the ROR program team.
Cette réponse vous a-t-elle été utile ?
There are several levels of requirements for a module’s integration into the Hospital Information System.
The main requirements set out by the referential are:
- Interfacing, control, security guidelines;
- Prescription process requirements;
- National prescription thesaurus integration requirements;
- Requirements regarding medico-economic and decision processes;
- Guidelines for ergonomics, functions, and notification alerts;
- Settings function requirements.
In total, 139 requirements are used to reach the minimal level of security needed for a solution’s integration into the hospital information system.
Cette réponse vous a-t-elle été utile ?
The current referential (2017) was written with industry experts. It introduces the particular context of this mission and the goals to reach in order to improve safety measures in neonatology and paediatric reanimation. The referential walks you through the fundamental concepts you need to grasp to understand the several requirements and protocols involved with software development for this sector.
Cette réponse vous a-t-elle été utile ?
Neonatology is a high-risk practice for two main reasons:
- The patients are extremely fragile (premature babies)
- 50% of the drugs used in the sector have yet to receive a marketing authorisation.
A survey conducted in 2014 evidenced a risk in the prescription process across the sector. The digitalisation of prescription is among the 41 recommendations that the report issued in order to increase safety levels.
Cette réponse vous a-t-elle été utile ?
PLATINES is a platform dedicated to interoperability tests for eHealth. It stimulates a ROR (version 2.4) and gives you the opportunity to use “test” data via several professional cases (a total of 94 test scenarios are available). These test scenarios are valid for the current ROR as well as the national ROR.
You must contact the ROR program team to request access to PLATINES.
Cette réponse vous a-t-elle été utile ?
In order for your service to be referenced in the My Health Space database, it must comply with the eHealth doctrine.
The doctrine is re-edited each year and defines the base rules of good practice for security, interoperability, and ethics applicable to eHealth services.
Cette réponse vous a-t-elle été utile ?
My Health Space includes the following health data:
- The medical profile entered by the patient, in an unstructured format (preconditions, allergies);
- Health measurements (weight, height, BMI, temperature, waist size, heart rate, blood pressure, glycemic index, level of pain) in FHIR format;
- Health documents (for example: test and diagnosis results, hospitalisation reports, medical treatments).
Cette réponse vous a-t-elle été utile ?


