Votre question concerne quel type d'offre ?
Votre question concerne quel couloir Ségur ?
Votre question concerne quel dispositif Ségur ?
Votre question concerne quel produit ou service produit?
Votre question concerne quelle thématique ?
The cost is determined by the certifying body which grants the certificate.
The daily cost to conduct the audits is at their discretion. The industrial is free to choose which certifying body they want to work with to become certified.
For further information on this topic, we suggest you contact the certifying bodies that have signed a convention agreement with the ANS, listed below:
Cette réponse vous a-t-elle été utile ?
Health professionals are encouraged to use MOS and NOS for three main reasons :
- enhance the interoperability of information systems by harmonising names, attributes, codes and nomenclatures;
- share the same understanding of the information, regardless of which directory or reference document it is taken from;
- facilitate the specification, analysis and conception of a project.
The ANS offers training on MOS-NOS and the elaboration method for exchanges’ functional specs (see our Documentation section).
The illustration below gives you examples of the types of professionals using the MOS-NOS:
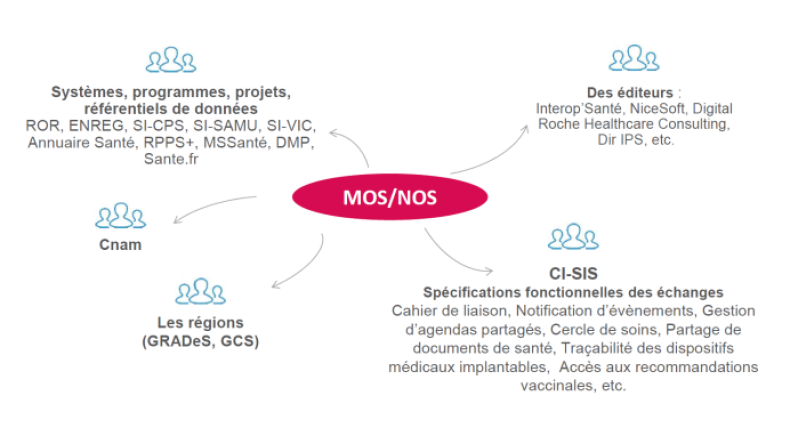
Cette réponse vous a-t-elle été utile ?
The MOS is a collection of concepts described homogeneously and neutrally in terms of technologies. It offers a common description of the information processed and exchanged in the information systems to make digital communication easier.
The overall consistency of the MOS is founded on the definition and description of its UML (Unified Modelling Language) concepts.
Some MOS concepts can be coded. They are associated with the nomenclatures of health objects (NOS), i.e. lists of codes/labels.
You are encouraged to use MOS and NOS to :
- optimise and coordinate efforts when you analyse or conceive a system (or an application) by re-using the same semantic components;
- make sure there is consistency in your internal developments and with external systems, for the best possible interoperability.
Cette réponse vous a-t-elle été utile ?
The European Commission’s studies have determined there is a need for a base vocabulary, such as MOS and NOS, which can be used as a starting point for:
- develop and evolve information systems (IS) to formalise the conceptual and logical data they utilise (for instance, the MOS is a reference for the modelling of the RPPS);
- share information between ISs to create specific models of data they can use (thus, the MOS is underlying the ROR exposure model);
- combine and synthetise elements originating from different sources;
- publish data in a common format, such as a directory or catalogue of service (for example, the Annuaire Santé / Health Pro Directory).
Cette réponse vous a-t-elle été utile ?
Yes, you need to have the ISO 9001 or the ISO 13485 certification to be eligible. You may begin the QHN certification process while you are in the course of making an application for/or renewing these ISO 9001 or the ISO 13485 certifications.
Cette réponse vous a-t-elle été utile ?
Yes, as long as the industrial remains responsible for the compliance of these processes with the requirements set out to obtain the QHN certification – regardless of hired external contractors or if some of the components are published or made by third-party companies.
Indeed, in the whole production system’s organisation, the industrial is the direct contact with a health professional or structure, and must make sure each requirement set out in the “Référentiel Qualité Hôpital Numérique” (QHN Referential, available in our Documentation section) is met.
Cette réponse vous a-t-elle été utile ?


