Qu’est-ce que le Référentiel de Néonatalogie ?
Développer la sécurité en néonatologie et réanimation pédiatrique fait partie des priorités de l'ANS. Un cahier des charges a été établi pour définir un référentiel de bonnes pratiques dans ce secteur à la patientèle fragile.
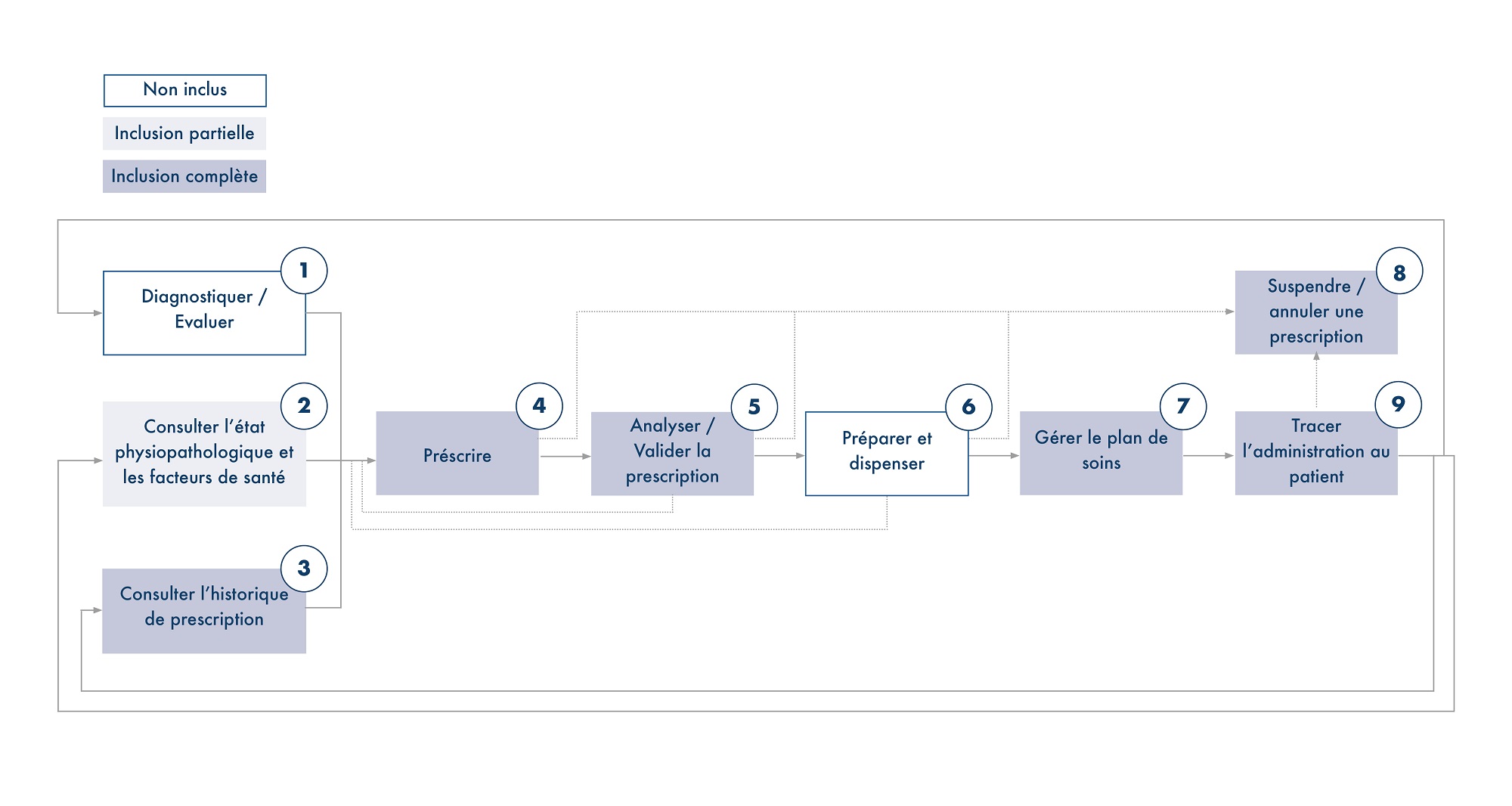
Le manque d’offres de solutions numériques en néonatologie et réanimation pédiatrique constitue aujourd’hui un obstacle aux besoins métier dans ce domaine. Il faut donc formaliser les fonctionnalités nécessaires dans un référentiel de néonatologie afin de couvrir le processus complet, et d’encourager l’essor de solutions e-santé pour cette patientèle. En première ligne : une forte demande de solutions logicielles pour la gestion du processus, de la prescription à l’administration en réanimation pédiatrique et en néonatologie.
L’ANS veut optimiser le niveau de sécurité en néonatologie. Or, plusieurs facteurs rendent ce processus délicat. En tant qu’industriel, ces enjeux vous concernent aussi.
Notre mission est de vous aider à :
- adapter les logiciels d’aide à la prescription : conçus pour la patientèle adulte, ils sont inadaptés en néonatologie ;
- harmoniser les pratiques observées, pour faciliter le développement d’offres standards ;
- simplifier les problématiques de gestion, par exemple dans la fonction de prescription des sites hospitaliers ;
- définir un nouveau référentiel pour la certification obligatoire des logiciels hospitaliers ;
- cibler un marché de niche, avec moins de 100 unités actives en France.
Le référentiel de néonatalogie en 1 clic
Modélisation du processus métier concerné par le référentiel de néonatologie
Le référentiel de néonatologie en quelques chiffres
75 unités de néonatologie en France en 2015 – un marché de niche à développer
41 préconisations de l’Inspection Générale des Affaires Sociales (IGAS) pour sécuriser le secteur néonatalogie et réanimation pédiatrique
139 exigences à remplir pour atteindre le niveau minimal de sécurité requis dans l’intégration d’un module avec les SIH








